The RightMed® Oncology Test
Personalize treatment and reduce toxicity risk based on patients’ genes with RightMed® Oncology tests1,2
You already personalize treatment with tumor profiling – raise your standard of care to include pharmacogenomic (PGx) guided prescribing for chemotherapy and supportive care medications. RightMed for Oncology empowers you to tailor medications and dosages to match the unique genetic makeup of individual patients based on established guidelines.
OneOme can help you integrate PGx into your health system – with Clinical Decision Support, clinician education, member communication materials, billing options and PGx program design consulting.

5-FU + Ondansetron + Codeine
Will these medications be safe and effective for your patient?
The RightMed Oncology test will provide you insights into how your patient may metabolize dozens of medications with PGx guidance used for chemotherapy and supportive care – including mental health, pain management and chemotherapy induced nausea and vomiting.
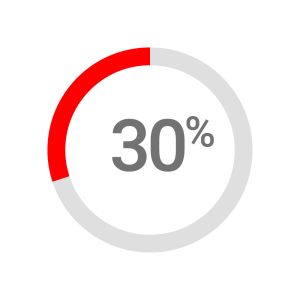
Nearly 30% of patients treated with fluoropyrimidines experience severe toxicity – in some cases, toxicity can be fatal3

60% of oncology patients are prescribed 1+ supportive care medication with PGx guidelines4
Click to learn more about the FDA safety and warning label changes for fluorouracil injection products (3/21/24).5
Studies show PGx may reduce risk without treatment delays

Reduced Risk for DPYD
PGx-guided prescribing in patients carrying the DPYD *2A allele reduced the risk of fluoropyrimidine-induced grade >/=3 toxicity to 28% compared to 73% in historical controls – drug-induced death reduced from 10% to 0%1

Reduced Risk for UGT1A1
UGT1A1 poor metabolizer (PM) patients received a median 30% dose reduction of irinotecan, and the incidence of febrile neutropenia was 6.5% compared to 24% in historical UGT1A1 PMs receiving full dose therapy (P = 0.04).2

Quick Turn-Around-Time
75+% of patients had results prior to initiation of chemotherapy…with a median TAT of 7 calendar days6

RightMed Oncology test portfolio
The RightMed oncology portfolio empowers innovative health systems, oncology practices and prescribers to be as focused or comprehensive as their oncology program requires. OneOme offers single and double-gene RightMed tests, as well as the RightMed Oncology panel test. The original RightMed test, which is even more comprehensive, is also available.
RightMed Oncology Panel Test
New germline PGx test focused on medications and genes with relevant guidance for the prevention of chemotoxicity or to be paired with somatic testing in chemotherapy and supportive care medications – including mental health, pain management and chemotherapy-induced nausea and vomiting.
Single Gene Tests
DPYD – test covering 5 alleles – *2A, *13, HapB3, c.2846A>T and c.557A>G – associated with fluorouracil and capecitabine.
UGT1A1 – single gene test associated with irinotecan.
TPMT/NUDT15 – double gene test associated with thiopurines.
Ochsner Health + OneOme Oncology PGx Program
“Prior to instituting a pharmacogenomics program, we had over 100 hospital days each year due to DPYD deficiencies. Patients who came in with severe toxicities from 5-FU chemotherapy.
Today we have virtually none.”
– Dr. Marc Matrana, Ochsner Health
PGx Program Support
Clinical Decision Support
Vantage interactive Clinical Decision Support tool provides clinicians easy access to a patient’s RightMed test results, including genetic information and insights on dozens of medications based on the patient’s genetic profile. We also offer clinician education materials, webinars and/or on-site training.
Evidence Based Testing
All RightMed tests are selectively curated by a group of pharmacists and scientists using OneOme’s rigorous standards for pharmacogenomic evidence. Each sample is analyzed at OneOme’s CLIA-certified, CAP-accredited in-house laboratory. Current turn-around-time is 4-5 days.
Program Design
OneOme can help you design and implement a PGx program at your health system or oncology group practice. From EHR integration to workflow design to invoicing options — contact us to learn more about how we collaborate with our partners.
1. Deenen MJ, Meulendijks D, Cats A, et al. Upfront genotyping of DPYD*2A to individualize fluoropyrimidine therapy: a safety and cost analysis. J Clin Oncol. 2016;34(3):227-34.
2. Hulshof EC, de With M, de Man FM, et al. UGT1A1 genotype-guided dosing of irinotecan: A prospective safety and cost analysis in poor metaboliser patients. Eur J Cancer. 2022;162:148-57.
3. Lesnjakovic L, Ganoci L, Bilic I, et al. DPYD genotyping and predicting fluoropyrimidine toxicity: where do we stand? Pharmacogenomics. 2023 Jan;24(2):93-106.
4. Shugg T, Ly RC, Rowe EJ, et al. Clinical opportunities for germline pharmacogenetics and management of drug-drug interactions in patients with advanced solid tumors. JCO Precis Oncol. 2022 Feb:6:e2100312.
5. FDA approves safety labeling changes regarding DPD deficiency for fluorouracil injection products, available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-safety-labeling-changes-regarding-dpd-deficiency-fluorouracil-injection-products. (March 21, 2024).
6. Muldoon M, Beck M, Sebree N, et al. Real-world implementation of DPYD and UGT1A1 pharmacogenomic testing in a community-based cancer center. Clin Trans Sci. 2024 Feb; 17(2): e13704.
What you should know before considering the RightMed Test or any genetic test:
- The RightMed Test is indicated for use in adults.
- The RightMed Test must be ordered by a licensed healthcare provider.
- The RightMed Test, as with all genomic testing, has limitations. Patients should always discuss the test with a healthcare provider knowledgeable about the test to see if it’s right for them.
- RightMed Test results are not a substitute for medical advice and should only be used in consultation with a medical professional.
- The RightMed Test does not determine the best medication for the patient; it is intended to inform healthcare providers of pharmacogenetic information and professional guidelines associated with the detected genotypes and predicted phenotypes.
- Independent healthcare providers available through OneOme’s service will not have all of the patient’s health history, which may cause the test results to be subject to a different interpretation than by the patient’s personal physician.
- The RightMed Test is not designed for diagnosis of any condition or disease. For more information on appropriate use, please read our INDICATIONS FOR USE.
- PATIENTS: DO NOT MAKE ANY CHANGES TO YOUR CURRENT MEDICATIONS OR DOSING WITHOUT CONSULTING YOUR HEALTHCARE PROVIDER.
